Erlotinib: what is the basic mechanism?
Erlotinib, the trade name for isotretinoin, is a drug used to treat certain forms of non-melanoma cancer that has spread from the primary tumor to neighboring tissues or even to other areas of the human body. This type of cancer accounts for five percent of all non-cancer skin cancer cases. It is usually effective in the treatment of cancers of the breast and lung. It was originally approved by the US Food and Drug Administration (FDA) in 1997 for the treatment of pre-neoplastic breast cancers in both men and women. It has since been approved for use in several additional countries.
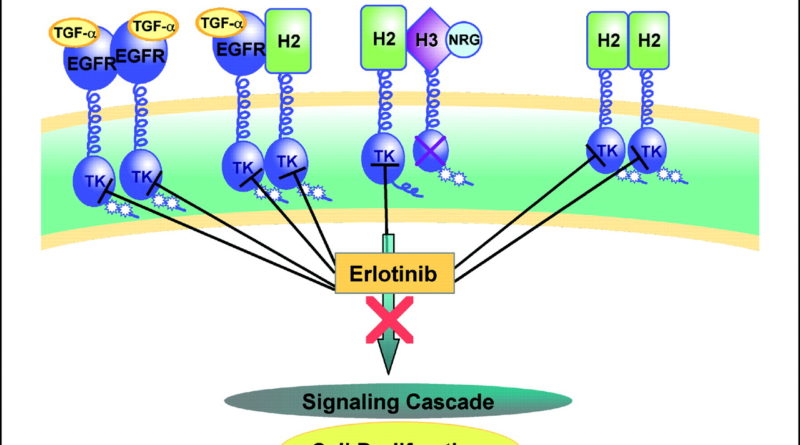
The basic mechanism of action of erlotinib at https://www.aasraw.com/products/erlotinib/ involves inhibition of the phosphodiesterase (PDE) enzyme. The enzyme is important in the recognition, synthesis, and elimination of various chemicals, including amino acids, sugars, fatty acids, and steroids.
As part of the metabolism of these chemicals, the PDEs are inhibited by an enzyme called tyrosine kinase domain. If the tyrosine kinase domain is not inhibited, it will release the chemical substrate, which in turn stimulates the growth of cancer cells. To protect against resistance, erlotinib can be administered to patients whose cancer cells have a high rate of division.
Patients taking erlotinib for treatment of lung adenocarcinoma have responded very well to this drug, particularly with the use of erlotinib (also known as trifluoperazine) in combination with chelators (egrulose and heparin). Because of its ability to target only non-cancerous tumors in the lung, the most common form of treatment is lung adenocarcinoma in single patients.
Although there are some reports of successful treatment of primary tumors, patients receiving chelators or trifluoperazine for the purpose of erlotinib should have regular clinical examinations, as there are possible interactions between these drugs and erlotinib.
In addition to lung disease, trifluoperazine is sometimes combined with other drugs to enhance its effect. For example, in combination with chelators, erlotinib can be administered to patients diagnosed with disseminated cell carcinoma (DCCC), palliative care for early secondary cancers in the chest (ppedarotitis), and ulcerative colitis (UC).
While it is unlikely that the combination of these drugs will prevent or cure DCCC or palliative care for early secondary cancers, patients receiving erlotinib may experience an improvement in symptoms and a reduction in systemic enzyme levels.
Because the PDEs play such an important role in metabolism, many doctors may decrease the recommended dose of Erlotinib like Neratinib for treatment of lung disease or ulcers by approximately 50%. When this happens, the patient may need to receive two doses of the medication instead of one.
The reason for this is that the additional dose of Erlotinib increases its effectiveness against the bacteria and reduces its potential for interaction with other medications. Patients who are receiving this type of adjustment to reduce their Erlotinib dosage should not expect an increase in the number of allergic reactions or skin rash. This would be expected only if the additional dose of Erlotinib were administered alone.